What We Do
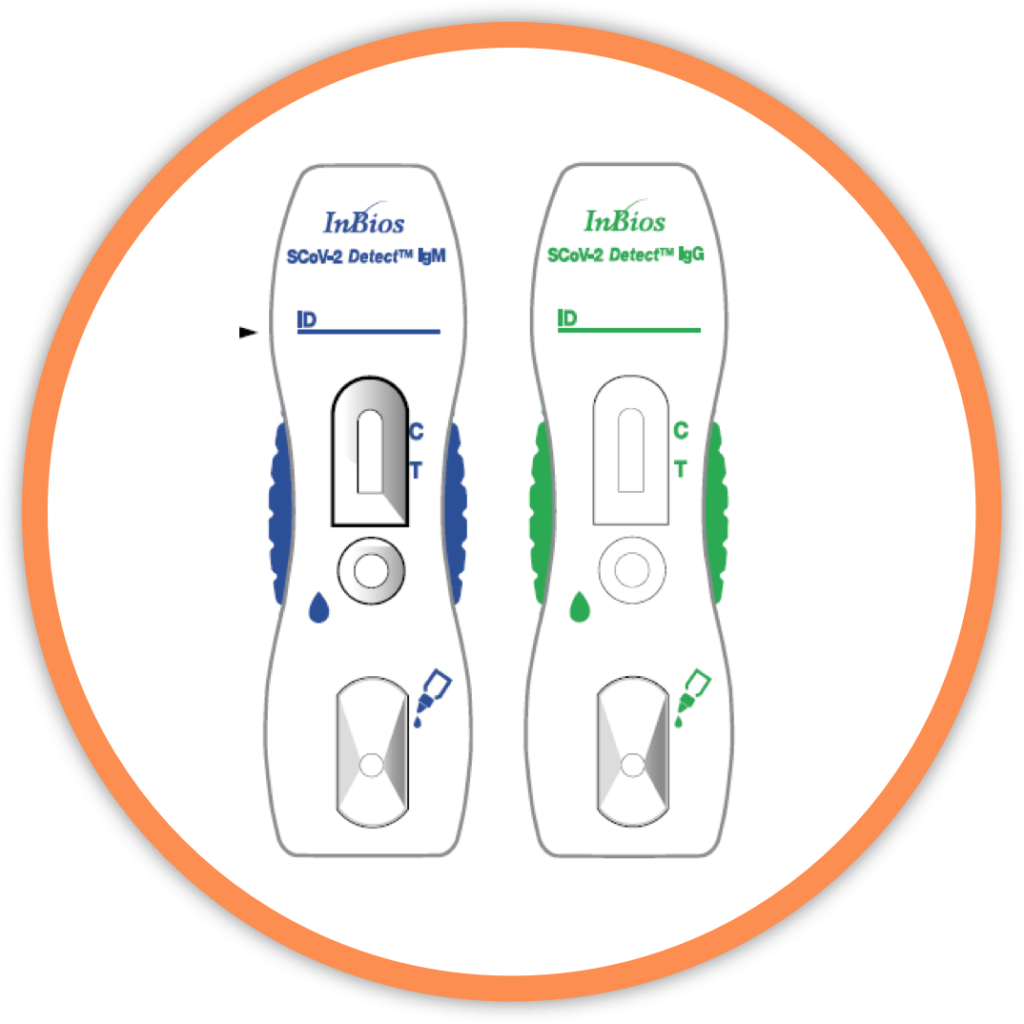
InBios improves health outcomes by providing superior quality diagnostic products
that are accurate, easy to use and cost effective.
How InBios Products Improve Health Outcomes
Experience
Privately owned since our founding in 1996, InBios has a talented team of scientists and engineers with over 25 diagnostic products and an extensive catalog of life science reagents on the market. Our committed leadership team drives innovation to continuously raise the standard of quality for our products.

Superior Quality
InBios has nearly 25 years of experience and an unwavering commitment to quality. All products are manufactured in our state-of-the-art facility in Seattle, WA, USA, and we are GMP compliant, FDA registered, USDA licensed and ISO 13485:2016 certified. This commitment to quality results in products that are accurate and user friendly.

Responsiveness to Emerging Diseases
With our expertise in emerging diseases and biothreats, our staff can readily respond to the latest infectious disease threat. We have proven success in navigating the regulatory pathways to react quickly and speed products to clinical labs to meet urgent diagnostic demands.
Cost Effectiveness
While quality is of utmost importance, InBios also strives to produce products that are cost effective, an important factor in serving the global marketplace where fast, easy-to-use diagnostics are critical.