InBios® COVID-19/Flu A&B RespiraDX™ Rapid Test
US FDA 510(k) Cleared • IVD • CLIA-waived • Point-Of-Care
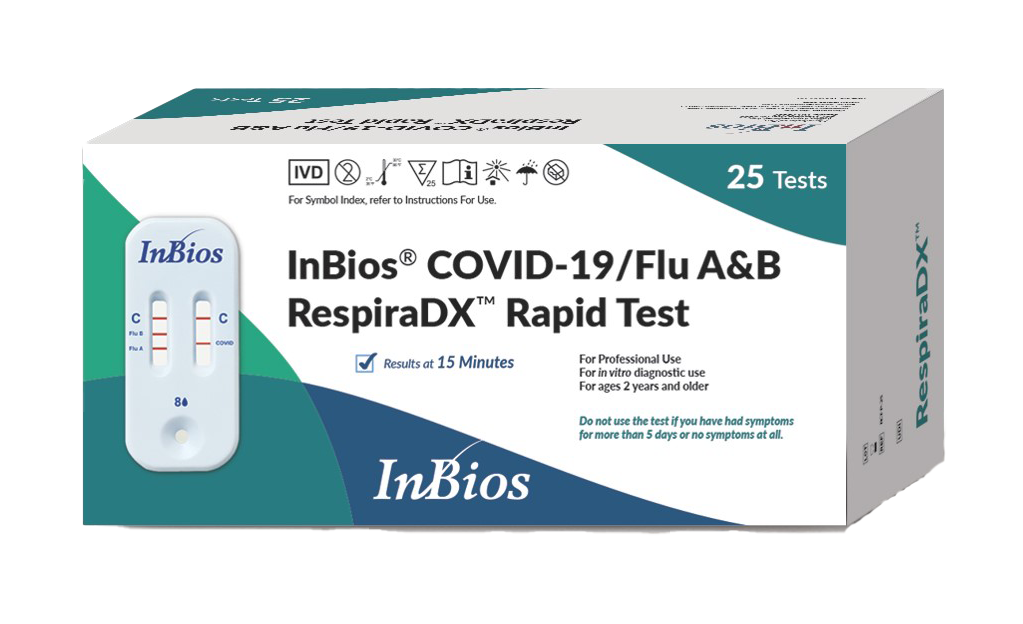
The InBios® COVID-19/Flu A&B RespiraDX™ Rapid Test is a lateral flow immunochromatographic assay intended for the qualitative detection and differentiation of influenza A, and influenza B nucleoprotein antigens and SARS-CoV-2 nucleocapsid antigen directly in anterior nasal swab samples from individuals with signs and symptoms of respiratory tract infection.
See Instructions For Use below for full intended use.
Resources
For more information, download the instructions for use via the “Downloads” tab.