Let’s Keep Each Other Healthy!
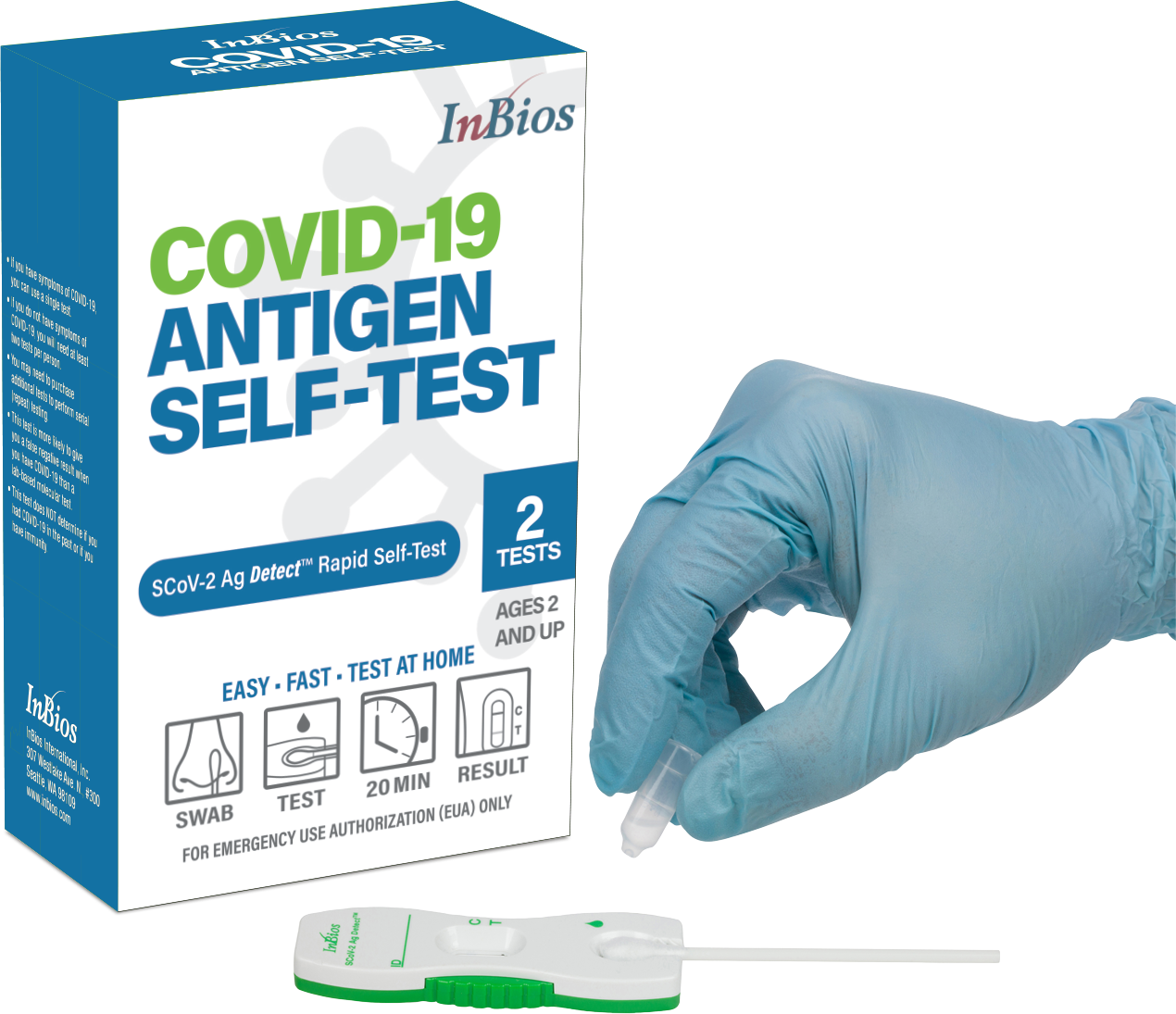
Easy, Fast, At-Home Testing for COVID-19
SCoV-2 Ag Detect™ Rapid Self-Test
How it Works
Uniquely Designed for Simplicity
Directly test your nasal swab sample.
No mixing needed!
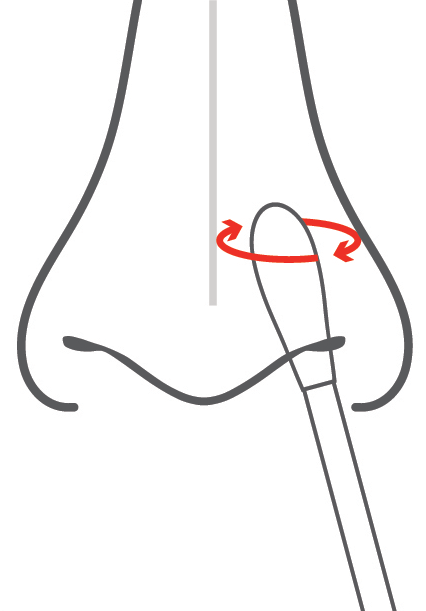
Swab
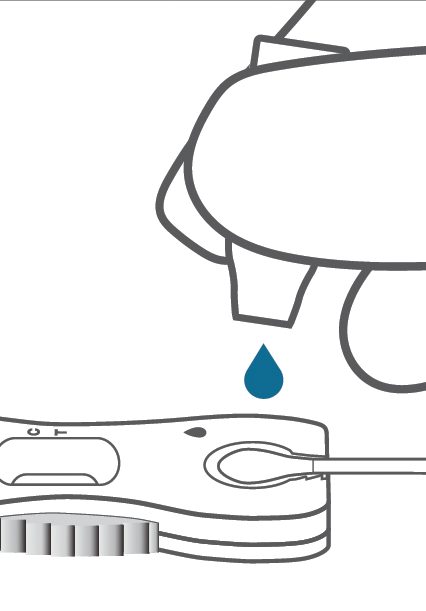
Test
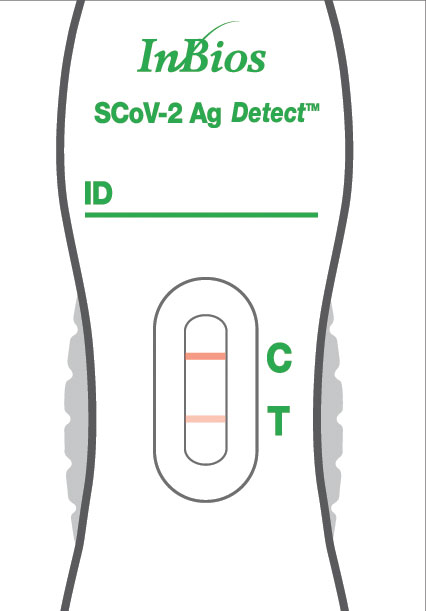
Read Results
See Instructions for Use for a step-by-step guide to performing the test.
Fast, Easy, Test at Home
The SCoV-2 Ag Detect™ Rapid Self-Test’s patent pending design has been developed to be incredibly simple to use. It uses a shallow nasal swab (inserted just one-half inch) and offers easy-to-read results – all in the time it takes to drink your morning latte, go for a mile run or read a couple of chapters in your latest novel.
FSA or HSA eligible
Study shows InBios’ test detects SARS-CoV-2 variants
Patent pending ease-of-use design
Uses minimally invasive shallow nasal swab
Simple positive or negative visual read – no instrument required
Can be used by individuals 14 years and older or with adult-collected nasal swabs from children as young as 2 years old
Can be used by individuals with or without symptoms.
Determining a negative result requires multiple tests. You may need to purchase additional tests to perform serial (repeat) testing. See instructions for use for complete details.
PPA (Relative Sensitivity) 85.71%; NPA (Relative Specificity) 100%
What We Do
InBios improves health outcomes by providing superior quality diagnostic products
that are accurate, easy to use and cost effective.
Featured News & Events
See how the tests works.
Resources
SCoV-2 Ag Detect™ Rapid Self-Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from the SARS-CoV-2 virus.
This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 or older or adult collected anterior nasal (nares) swab samples from individuals aged 2 years or older. This test is authorized for individuals with symptoms of COVID-19 within the first 5 days of symptom onset when tested at least twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested at least three times over five days with at least 48 hours between tests.
The SCoV-2 Ag Detect™ Rapid Self-Test does not differentiate between SARS-CoV or SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen, which is generally detectable in anterior nasal (nares) samples during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses and the agent detected may not be the definitive cause of disease. Individuals who test positive with the SCoV-2 Ag Detect™ Rapid Self-Test should self-isolate and seek follow up care with their physician or healthcare provider as additional testing may be necessary.
All negative results are presumptive and confirmation with a molecular assay, if necessary for patient management, may be performed.
Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control measures such as isolating from others and wearing masks. Negative results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19.
Individuals who test negative and continue to experience COVID-19 like symptoms of fever, cough and/or shortness of breath may still have SARS-CoV-2 infection and should seek follow up care with their physician or healthcare provider.
Individuals should provide all results obtained with this product to their healthcare provider for public health reporting and to receive appropriate medical care. All healthcare providers will report all test results they receive from individuals who use the authorized product to relevant public health authorities in accordance with local, state, and federal requirements, using appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Page 2 of 19 Mapping for SARS-CoV-2 Tests provided by CDC.
The SCoV-2 Ag Detect™ Rapid Self-Test is intended for non-prescription self-use and/or as applicable, for an adult lay user testing another aged 2 years or older in a non-laboratory setting. The SCoV-2 Ag Detect™ Rapid Self-Test is only for in vitro diagnostic use under the Food and Drug Administration’s Emergency Use Authorization. This product has not been FDA cleared or approved.
For information on distribution, contact us here.
- Healthcare Provider Instructions
- Quick Reference Instructions (English)
- Quick Reference Instructions (Spanish)
- FDA Letter of Authorization
- FDA Shelf Life Extension 13 Months
- FDA EUA210619/S010 Serial/Repeat Testing Revision 12.23.22
- Fact Sheet for Healthcare Professionals
- CN-0009 Change Notice-FDA Intended Use-Serial Testing Updates
- SDS-0075-00 US SCoV-2 Ag Detect Rapid Self-Test Kit
Frequently Asked Questions
SCoV-2 Ag Detect ™ Rapid Self-Test
InBios received an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the SCoV-2 Ag Detect™ Rapid Self-Test on Nov. 22, 2021.
It is a test to detect proteins (called antigens) from the virus that causes COVID-19. A positive result means that it is very likely you have COVID-19, and it is important to be under the care of your healthcare provider. The test can be performed using shallow nasal swab samples, requires no mixing step, and takes ~20 minutes to obtain results. This test is authorized for non-prescription home use.
No. It is authorized for over-the-counter use.
Insurance may cover some or all of the cost of your test. Please consult with your specific health insurance provider to determine if your test will be covered. This product is FSA or HSA eligible. Reimbursement and free-of-charge tests are not provided by InBios. Additional information on coverage and reimbursement can be found at this link to the Centers for Medicare and Medicaid.
The SCoV-2 Ag Detect™ Rapid Self-Test is available in a 2 tests per kit configuration. Each kit includes everything you need to perform 2 tests (swabs, test cassettes and dropper bottles) as well as detailed instructions for use. An instructional video is also available here.
Thirteen months from the date of manufacture.
This test can be used by individuals aged 14 years and older or with adult-collected nasal swabs from children as young as 2 years old.
The SCoV-2 Ag Detect™ Rapid Self-Test’s patent pending design has been developed to be incredibly simple to use, even for someone who has not self-tested before. It utilizes a nasal swab (inserted just one-half inch) to keep discomfort at a minimum.
No, this test requires no additional instrumentation and results can be read visually. You will need a timer or stopwatch to time the test.
Your results will be ready in 20 minutes. Do not read results before 20 minutes or after 25 minutes.
No, the nasal swab is not sharp, and it should not hurt. Sometimes the swab can feel slightly uncomfortable. If you feel pain, please stop the test and seek advice from a healthcare provider.
Potential risks include possible discomfort during sample collection and/or possible incorrect tests results (refer to the “Understanding Your Test Results” section of the instructions). Potential benefits include helping your healthcare provider to make informed recommendations about your care and helping limit the spread of COVID-19 within your family and community.
The test is positive if a control line and a test line both show on the cassette. Be sure to look closely – even a faint line indicates a positive result. The test is negative when only the control line appears. The test is invalid if no control line appears, whether or not there’s a test line.
You do not need to perform repeat testing if you have a positive result at any time.
A positive result means that it is very likely you have COVID-19 because proteins from the virus that causes COVID-19 were found in your sample. You should self-isolate from others and contact a healthcare provider for medical advice about your positive result.
To increase the chance that the negative result for COVID-19 is accurate, you should:
- Test again in 48 hours if you have symptoms on the first day of testing.
- Test 2 more times at least 48 hours apart if you do not have symptoms on the first day of testing.
A negative test result indicates that antigens from the virus that causes COVID-19 were not detected in your sample. However, if you have symptoms of COVID-19, and your first test is negative, you should test again in 48 hours since antigen tests are not as sensitive as molecular tests. If you do not have symptoms and received a negative result, you should test at least two more times with 48 hours in between tests for a total of three tests. If you have a negative result, it does not rule out SARS-CoV-2 infection; you may still be infected and you may still infect others. It is important that you work with your healthcare provider to help you understand the next steps you should take.
If no control line shows up on the test, the result is invalid (even if any test line shows up). An invalid result means the test was not able to tell if you have COVID-19 or not. If the test is invalid, a new swab should be used to collect a new nasal specimen and the test should be run again, using a new test and dropper bottle.
The test can be used by individuals with or without symptoms. Determining a negative result requires multiple tests. You may need to purchase additional tests to perform serial (repeat) testing. See instructions for use for complete details.
Serial (repeat) testing is when a single person is tested for COVID-19 more than once. Because antigen tests are less sensitive than other COVID-19 tests and false results may occur, repeated testing may identify more individuals with COVID-19 infection than a single test. By repeating testing, it may be possible to identify cases of COVID-19 infection and reduce spread of infection more quickly. Additional testing with a molecular COVID-19 test may be necessary, depending on your individual risk factors and test results. It is important that you work with your healthcare provider to help you understand the next steps you should take. Serial testing is more likely to detect COVID-19, especially when you do not have any symptoms. Determining a negative result requires multiple tests. You may need to purchase additional tests to perform serial (repeat) testing. See instructions for use for more details.
An antigen test, such as the SCoV-2 Ag Detect™ Rapid Self-Test, detects proteins from the virus. Molecular tests detect genetic material from the virus. Antigen tests are very specific for the virus, but not as sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not rule out infection. If your test result is negative, you should discuss with your healthcare provider about whether an additional test is necessary and if you should continue isolating at home. There is a higher chance of false negative results with antigen tests than with laboratory-based molecular tests. This means that there is a higher chance this test will give you a negative result when you have COVID-19.
No. Individuals can utilize this test regardless of vaccination status.
The SCoV-2 Ag Detect™ Rapid Self-Test does not provide documentation of a test result for this purpose. Please visit the website of your airline for up-to-date information about what is required. The CDC also provides information about requirements for international travel.
Please recycle the box (it is 100% recyclable), but dispose of the swab, test cassette, and dropper bottle in common household waste.
In a study published in JAMA Network Open, SCoV-2 Ag Detect™ Rapid Self-Test demonstrated similar analytical and clinical performance across 3 phases of circulating SARS-CoV-2 variants (pre-Delta, Delta, and Omicron). Sequence analysis performed at InBios also suggests our test will detect variants currently in circulation. We routinely monitor sequences deposited in GISAID and Nextstrain for emerging SARS-CoV-2 mutations and variants and pay special attention to CDC data on circulating rates, and variants defined by the CDC as Variants of Concern (VoC) and Variants of Interest (VoI). Performance at the time of testing may vary depending on the variants circulating, including newly emerging strains of SARS-CoV-2 and their prevalence, which change over time.
The SCoV-2 Ag Detect™ Rapid Self-Test is manufactured in the USA.
This product has not been FDA cleared or approved but has been authorized by FDA under an Emergency Use Authorization. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.
- For more information on EUAs, please visit http://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
- For the most up-to-date information on COVID-19, please visit cdc.gov/COVID19
Call 1-866-INBIOS1 or 206-344-5821 or submit an online inquiry.
This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.


