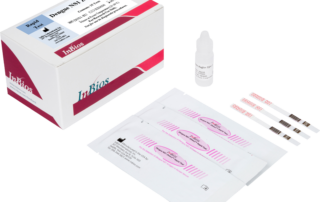
DENV Detect™ NS1 ELISA
FDA Cleared for In Vitro Diagnostic Use
The DENV Detect™ NS1 ELISA is for the early detection of Dengue virus (DENV) NS1 antigen in human serum. This test is for the presumptive clinical laboratory diagnosis of Dengue virus infection.

Resources
FDA Cleared & CE Marked
Early NS1 antigen detection (within first 7 days onset of symptoms)
Helps differentiate dengue from other flaviviruses that may cross react with dengue antibodies but not with the antigen
High Relative Sensitivity & Specificity: 86.6% PPA & 97.8% NPA with prospectively collected positive and negative confirmed clinical specimens
Kit can be performed in parallel with InBios’ FDA Cleared dengue IgM ELISA for presumptive diagnosis of acute or convalescent stage dengue virus
| Catalog No. | DNS1-1 |
| Format | Antigen Detection |
| Quantity/Kit | 96 wells/plate |
| Incubation Time | 60+30+20+1 |
| Sample Type | Serum |
| Storage | 2-8°C |
| Shelf Life | 18 months |
For more information, download instructions for use via the “Downloads” tab.
Related Products
Scrub Typhus Detect™ IgM ELISAinfo@inbios.com2023-10-11T16:08:17+00:00
Scrub Typhus Detect™ IgM ELISA
Scrub Typhus Detect™ IgG ELISAinfo@inbios.com2023-10-31T15:16:56+00:00