Kalazar Detect™ Rapid Test for Visceral Leishmaniasis
FDA Cleared for In Vitro Diagnostic Use
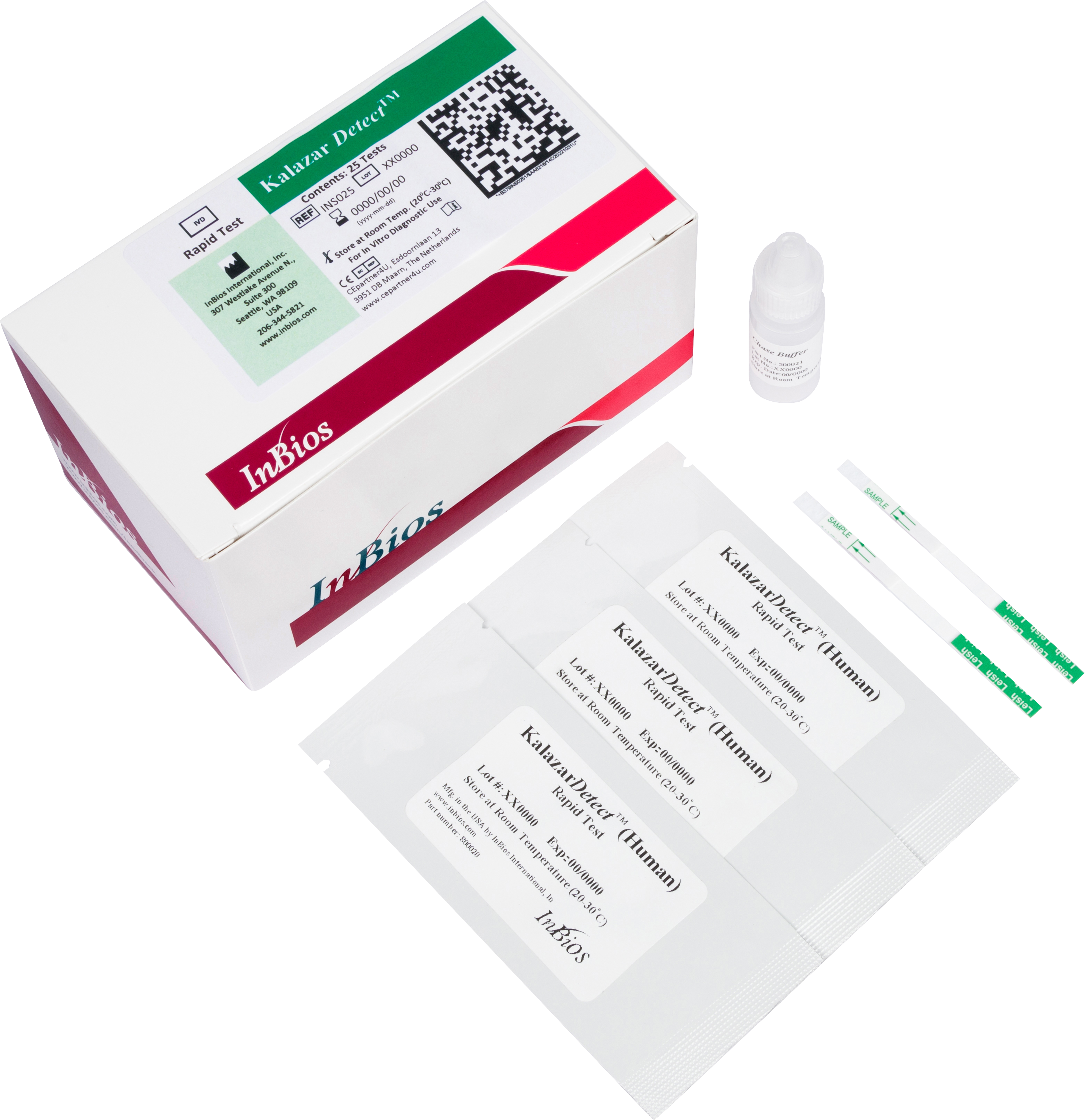
The Kalazar Detect™ Rapid Test for Visceral Leishmaniasis (VL) is a rapid immunochromatographic strip assay for the qualitative detection of antibodies to members of L. donovani in human serum to aid in the presumptive diagnosis of VL.

Resources
US FDA Cleared
CE Marked
Relative Sensitivity and Relative Specificity: >90%
Fast Results: 10 minutes
Field Friendly: Refrigeration and lab equipment not required
| Catalog No. | INS025 |
| Format | ICT (strip) |
| Quantity/Kit | 25 tests |
| Time to Result | 10 minutes |
| Sample Type | Serum |
| Storage | Room Temperature (20°C-30°C) |
| Shelf Life | 24 months |
For more information, download the instructions for use via the “Downloads” tab.
Related Products
Active Anthrax Detect™ Plus Rapid Testinfo@inbios.com2024-07-25T17:07:21+00:00
Active Anthrax Detect™ Plus Rapid Test
Scrub Typhus Detect™ IgG Rapid Testinfo@inbios.com2024-07-25T21:29:28+00:00
Scrub Typhus Detect™ IgG Rapid Test
Chagas Detect™ Plus Rapid Testinfo@inbios.com2025-07-03T19:19:41+00:00
Chagas Detect™ Plus Rapid Test
Active Anthrax Detect™ Plus Rapid Testinfo@inbios.com2024-11-08T19:12:13+00:00
Active Anthrax Detect™ Plus Rapid Test
Kalazar Detect™ Rapid Test for Visceral Leishmaniasisinfo@inbios.com2024-07-25T18:23:49+00:00
Kalazar Detect™ Rapid Test for Visceral Leishmaniasis
Scrub Typhus Detect™ IgM Rapid Testinfo@inbios.com2024-07-25T21:26:34+00:00
Scrub Typhus Detect™ IgM Rapid Test
Active Melioidosis Detect™ Rapid Testinfo@inbios.com2024-11-19T17:06:17+00:00
Active Melioidosis Detect™ Rapid Test
CL Detect™ Rapid Test for Cutaneous Leishmaniasisinfo@inbios.com2025-06-04T16:43:10+00:00