CL Detect™ Rapid Test for Cutaneous Leishmaniasis
FDA Cleared for In Vitro Diagnostic Use
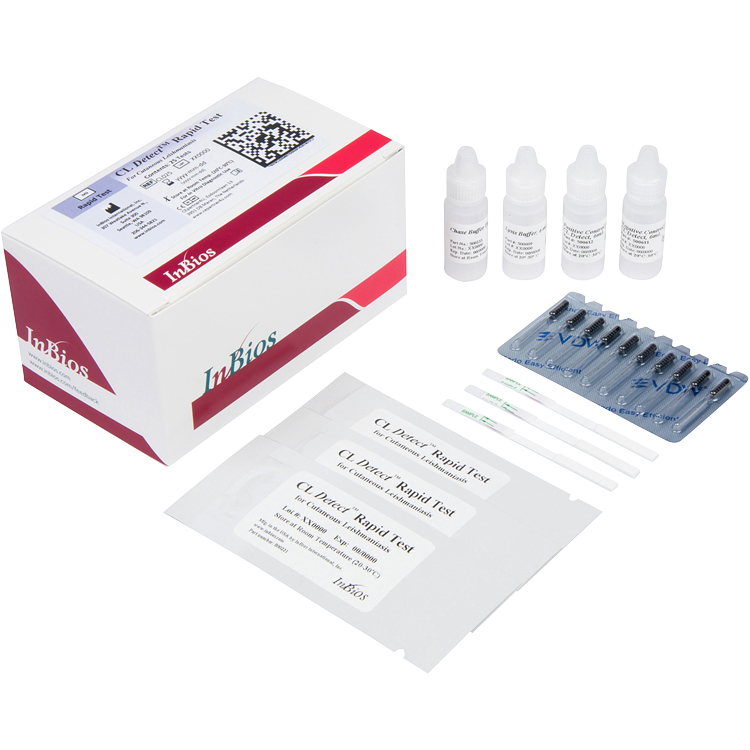
The CL Detect™ Rapid Test is a qualitative, in vitro immunochromatographic assay for the rapid detection of Leishmania species antigen in ulcerative skin lesions.

Resources
- US FDA Cleared
- CE Marked
Relative Specificity in non-endemic population: 96%
Relative Sensitivity and Specificity in endemic population: 100% and 84% respectively
Kit includes 25 tests, controls, buffers and sterile dental broaches
| Catalog No. | CL025 |
| Format | ICT (strip) |
| Quantity/Kit | 25 tests |
| Time to Result | 20 minutes |
| Sample Type | Skin lesions |
| Storage | Room Temperature (20°C-30°C) |
| Shelf Life | 24 months |
For more information, download the instructions for use via the “Downloads” tab.
Related Products
Active Anthrax Detect™ Plus Rapid Testinfo@inbios.com2024-07-25T17:07:21+00:00
Active Anthrax Detect™ Plus Rapid Test
Scrub Typhus Detect™ IgG Rapid Testinfo@inbios.com2024-07-25T21:29:28+00:00
Scrub Typhus Detect™ IgG Rapid Test
Chagas Detect™ Plus Rapid Testinfo@inbios.com2025-07-03T19:19:41+00:00
Chagas Detect™ Plus Rapid Test
Active Anthrax Detect™ Plus Rapid Testinfo@inbios.com2024-11-08T19:12:13+00:00
Active Anthrax Detect™ Plus Rapid Test
Kalazar Detect™ Rapid Test for Visceral Leishmaniasisinfo@inbios.com2024-07-25T18:23:49+00:00
Kalazar Detect™ Rapid Test for Visceral Leishmaniasis
Scrub Typhus Detect™ IgM Rapid Testinfo@inbios.com2024-07-25T21:26:34+00:00
Scrub Typhus Detect™ IgM Rapid Test
Active Melioidosis Detect™ Rapid Testinfo@inbios.com2024-11-19T17:06:17+00:00
Active Melioidosis Detect™ Rapid Test
CL Detect™ Rapid Test for Cutaneous Leishmaniasisinfo@inbios.com2025-06-04T16:43:10+00:00